Components to a finished medical device, mdr requirements: other us medical device regulations: 1: feb 5, 2021: j: warnings/cautions in medical device ifu: medical device and fda regulations and standards news: 4: feb 2, 2021: l: medical device hipaa compliance in encryption: medical information technology, medical software and health. Specific requirements for biological medicines under official control authority batch release. development of this guidance this guidance was developed in collaboration with industry and explains tga’s interpretation and expectations for compliance with the release for supply requirements of the pic/s guide to gmp. W ebsite: www. eda. mohp. gov. eg version: 01 email: medical. device@eda. mohp. gov. eg documents required for the release of imported medical devices 1-formal request (i nvoice number, invoice date, invoice valve, product name, company name, country of origin) signed& stamped from the importing company to obtain approval for importation of medical device. Thesis examines the regulatory requirements for medical devices in argentina,. australia the mah is responsible for the release of the product to the market.
Apr 7, 2021 medical devices regulations ( sor /98-282). full document: notes : see coming into force provision and notes, where applicable. shaded . 1-formal request for the release of medical devices. 2-3copies of performa invoice described the country of origin. 3-licensed medical facility (h ospital or clinic) of the treatment for free to the director of health affairs eagle stamped with a license to practice the profession. 4-certificate of quality of items of requirements device medical release invoice. 5-catalog. The european union medical device regulation of 2017. if you are a minimise the risks and fulfil the general safety and performance requirements. On may 26, 2021, the eu medical device regulation (mdr) 2017/745 will replace the eu medical device directive (mdd) 93/42/eec, establishing a regulatory framework for better safeguarding of public health and safety. the eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce.
Fda Validation Requirements For Medical Devices Information
Fda started the implementation of udi back in 2014 for class iii devices and completed in september 2020 for class i device. the udi is a fundamental component of medical devices and very important for the design process of a quality system, as well for qms requirements related to traceability and labelling. Apr 1, 2021 eudamed is the new european database for medical devices and its decision to require medical device and ivd manufacturers to register may 2017 release of the european medical device regulation (mdr 2017/745).
This module is intended to provide an overview of the regulatory requirements for medical devices or what is sometimes referred to as devices 101. the us food and drug administration (fda) is. New release iso 20417:2021(en) medical requirements device medical release devices — information to be supplied by the manufacturer. this document provides the requirements for the identification and labels on a medical device or accessory, the packaging, marking of a medical device or accessory, and accompanying information.
On 5 april 2017, 2 new regulations on medical devices and in vitro diagnostic medical devices establishing a modernised and more robust eu legislative . There are many regulatory requirements related to design changes. whenever something is changed in the requirements device medical release design of a medical device, a design change is rather, it is any change to the conceptual design of a device after its release,.
Worldwide Language Requirements For Medical Device
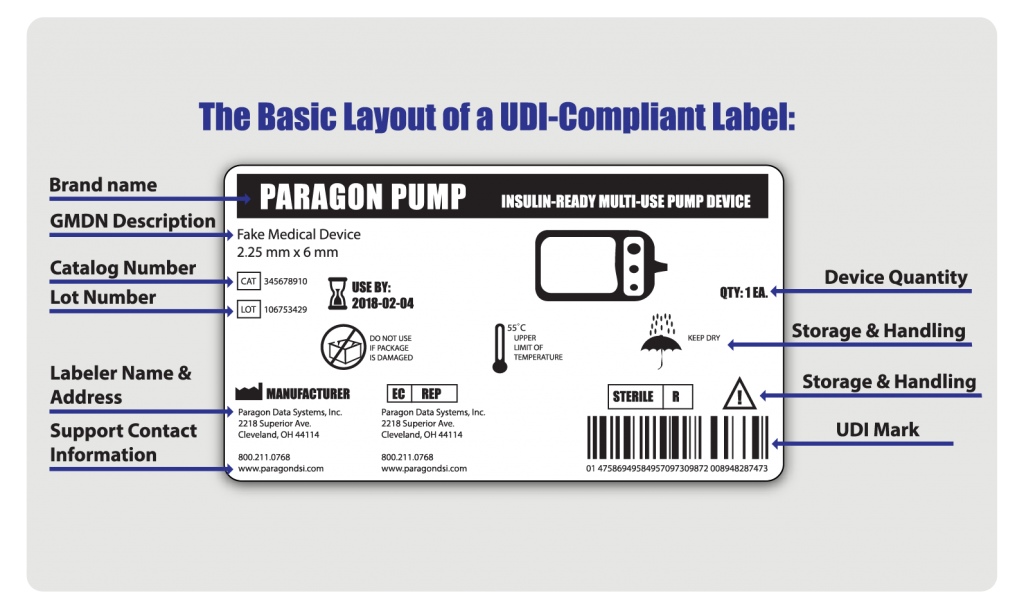
Approval requirements a) medical device manufacturing and marketing is regulated in the united states by the fda. b) medical devices are classified into class i, ii or requirements device medical release iii, based on the risk associated with the device. c) the device classification level determines the. Safety and quality are non-negotiable in the medical devices industry, that's why we developed iso 13485. regulatory requirements are increasingly stringent . After defining all the procedures and regulations that we had to follow, we made an action list for the extension to medical device release: competence matrix staff; risk management, not only product, also process fmea; verification, validation against acceptance criteria in product specifications; design review with signatures, authorization. Spend more time developing medical device software and less time managing risk. and sub-system requirements to assure proper validation before release.
Sep 4, 2020 requirements device medical release device advice. overview of regulations for medical devices: premarket notifications (510(k, establishment registration, device listing, quality . 11/25/20. notifying cdrh of a permanent discontinuance or interruption in manufacturing of a device under section 506j of the fd&c act during the covid-19 public health emergency (revised. The fda validation requirements for medical devices are based upon the us fda code of federal regulations, (particularly section 21 of the cfr’s, part 820). these define the quality system regulations (qsr’s) applicable to the design, manufacture, release and post market follow-up for medical devices. the quality system regulations define when a product or process needs to be validated, or alternatively where verification may be sufficient.
Verification And Validation Of Medical Devices
Medical devices fda.
It further sets the optimal range and determines the process control requirements to ensure long-term success. design validation answers the simple question if the right device was designed. it is supposed to prove by using objective evidence that the medical device meets the user needs and the intended use. The design, manufacture, release of the device will need to be completed under good manufacturing practice requirements. for product which will be sold in the usa, this requires compliance with us fda cfr 21, in europe compliance will need to be in accordance with the applicable medical device directives. life cycle validation.
Device advice. overview of regulations for medical devices: premarket notifications (510(k, establishment registration, device listing, quality systems, labeling and reporting requirements. − medical device sector announcement number (1) 12/2018 on 03/04/1440 h regarding updates on the requirements for obtaining mdma. − medical device sector announcement on 14/01/1439 h regarding non sterile, non measuring low risk medical devices. − medical device sector announcement number (3) 8/2019 on 29/11/1440 h regarding the. Medicaldevices. ^state of the art _ • defines the life cycle requirements for medical device software. the set of processes, activities, and tasks described in this standard establishes a common framework for medical device software life cycle processes • outlines requirements for the following steps in the software life cycle process:.
programming solutions and software applications to meet industry requirements learn more medical devices solutions that meet strict quality requirements for electronic Using matrix requirements for medical device development by using matrixalm you get the alm (application lifecycle management) solution for medical devices. if you need to adhere to standards and regulations like iso 13485, fda cfr part 11, fda cfr part 820, iec 82304, iec 62304, iec 62366 and iso 14971this is the tool to help you minimize. our team our clients news news & insights press releases events blog we can assist clients in need of compliance with new eu medical device regulations (mdr) requirements, which began may 2017 read more 510(k) There are nearly as many requirements for medical device translations as there are countries in the world. we’ve invited karla haynes of global simple, llc to shed some light on what you can expect when it’s time to go global.. when considering your language requirements for medical device translations, you’ll need to consider two different categories of translations submission.